Biojector
Centrul de Informare si Consiliere HIV/SIDA Noi si Ceilalti Bucuresti :: Informatii medicale - UPDATE
Pagina 1 din 1
 Biojector
Biojector
Fuzeon® (enfuvirtide, Roche) is indicated in combination with other antiretroviral agents for the
treatment of HIV-1 infected patients with evidence of HIV-1 replication despite ongoing antiretroviral
therapy and with few remaining therapeutic options (resistance or intolerance to the three main
antiretroviral classes: protease inhibitors, non-nucleoside reverse transcriptase inhibitors and
nucleoside reverse transcriptase inhibitors). As the only fusion inhibitor in its class, Fuzeon® has no
cross-resistance with the other classes of antiretrovirals. Thus, in 2007, Fuzeon® remains a useful
and sometimes necessary component of combination therapies for HIV patients with severe
therapeutic failure.
Unfortunately, patients taking Fuzeon® often face considerable difficulties due to its administration. As
you know, Fuzeon® requires two subcutaneous injections per day, and injection-site reactions (ISRs)
occur in almost all patients (1). These ISRs may include pain and discomfort, induration, erythema,
nodules and cysts, pruritus, and ecchymosis. They are often present at several injection sites. In some
patients, induration and nodules can remain painful during more than two or three weeks. With two
daily injections, even if the patients strictly comply with the precautions for use, they can rapidly have
their bodies covered with ISRs.
ISRs undermine the quality of life of HIV patients. They can reduce the ability of HIV patients to cope
with Fuzeon® injections and cause prejudice to patients whose viral load can rapidly grow without an
optimal adherence, which in turn can lead to therapeutic failure.
Roche, as Fuzeon® manufacturer, and Trimeris, as co-developer with Roche (2), are well aware that
ISRs caused by Fuzeon® are a major issue. Hence they decided to initiate – relatively soon after
Fuzeon® received marketing authorisation – the development of a needle-free device to deliver
Fuzeon®: the Biojector 2000®. This device uses the pressure generated by a CO2 cartridge to deliver
2 sur 6
the medication parenterally without the use of a needle.
Biojector 2000® has been shown to improve the subcutaneous dispersion of the injectant (3). This
may lead to reductions in the occurrence of pain and nodule formation that might be associated with
bolus deposition from subcutaneous administration with a needle.
Unfortunately, Roche and Trimeris recently decided to stop the co-development of Biojector 2000®
with Fuzeon®. They justify their decision by an analysis of the data collected from two clinical trials
they have sponsored during the three previous years: the WAND (4) and BOSS (3) studies. According
to Roche and Trimeris, « in these studies the device showed only a modest benefit for some patients
in terms of lessening signs and symptoms of ISR » (5). On the other hand, they point out that Biojector
2000® could be associated with severe adverse events, like « hematoma in patients with bleeding
disorders and nerve pain ». Moreover, they suggest that Biojector 2000® may lead to a loss of
enfuvirtide, hence a reduced efficacy of the drug: « In certain instances, improper use of the device
occurred such that following injection with Biojector, Fuzeon® was observed on the skin surface or
leaked back out of the skin. Whether this resulted in insufficient amounts of Fuzeon® being
administered with some injections is unknown. However, this would have the potential to affect
efficacy of the drug. »
We have analysed the abstracts that were published regarding the findings of WAND and BOSS as
well as the articles on Biojector 2000® and Fuzeon® published in medical journals (see annex 1).
Recently, we also met with Roche and contacted Canadian researchers involved in the Biojector
2000® trials. Taking into account the above-mentioned data, we disagree with the analysis made by
Roche and Trimeris.
Bioavailability of Fuzeon® with Biojector 2000®
In two pharmacokinetic studies (6), Biojector 2000® has been shown to have equivalent
exposure – Cmax, AUC and Cmin - and a similar general safety profile to needleadministration
of Fuzeon®.
Frequency and severity of ISRs with Biojector 2000®
Use of Biojector 2000® was associated with an improved ISR profile in enfuvirtideexperienced
participants in all the studies (3) (4) (6) (Annex 1). Use of Biojector 2000®
reduces significantly the incidence and severity of treatment-limiting ISRs (except for
ecchymosis) from Fuzeon® administration. Compared with needles, Biojector 2000® was
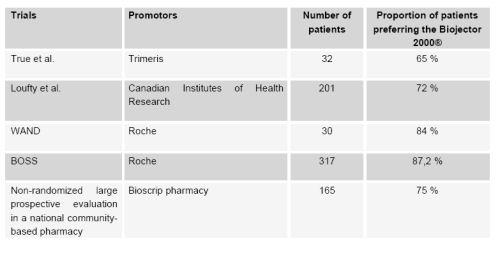
associated with an improved quality of life in patients taking enfuvirtide. In studies, a large
majority of participants preferred Biojector 2000® to the needle.
Centrul de Informare si Consiliere HIV/SIDA Noi si Ceilalti Bucuresti :: Informatii medicale - UPDATE
Pagina 1 din 1
Permisiunile acestui forum:
Nu puteti raspunde la subiectele acestui forum